Crystal structures of hybrid perovskites are not 0K
June 21, 2014
Most electronic structure techniques for materials modelling are athermal. Temperature is not treated (i.e. no zero point energy or vibrational entropy). The standard procedure is that all atomic forces (and cell stresses) are minimised to their ground-state configuration before properties are analysed.
It is possible to include temperature effects in various ways, e.g. molecular dynamics (Newtonian dynamics based on quantum mechanical forces) or lattice dynamics (harmonic or quasi-harmonic approximations). A good example of the latter approach is the thermal properties of lead chalcogenides that we published last month.
Perovskite (ABX3) structured materials are a particularly nasty (or interesting, depending on your level of intimacy) case in solid-state chemistry. A series of temperature driven phase transitions are observed based on movements of the structural building blocks. Usually the phase transitions involve rotation or tilting of the corner-sharing network of BX6 octahedra, which correspond to relatively small changes in atomic positions and lattice volumes. Describing and understanding the nature of these phase transitions has kept theorists and crystallographers in business for many decades. Most are second-order displacive transitions, where “soft" phonon modes are associated with ferroelectric or antiferroelectric instabilities.
It is common that at high temperatures a cubic perovskite structure emerges with beautiful octahedral symmetry (a theorist’s dream). Unfortunately, in most cases this structure is an average configuration, which does not represent the local structure at any particular moment in time. For example, Martin T. Dove commented on BaTiO3: “The Ti 4+ atoms appear to occupy a central site in the high temperature cubic phase only on average, whereas in practice that site is always a potential-energy maximum. The potential energy minima for the Ti 4+ cations are located away from the central site along the eight directions, so that in the high-temperature phase the Ti 4+ cations are hopping among the eight different sites." Nonetheless, for modelling one tends to impose the average space group symmetry which forces occupation of the potential energy maximum or saddle point. This “pseudo-cubic” structure leads to all sorts of peculiarities, e.g. if the symmetry constraints are broken through the formation of a point defect, a spontaneous phase transition can be observed.
I learned about the subtleties of these transitions working at UCL, where a PhD student supervised by Richard Catlow and Alexey A. Sokol was probing phase transitions in SrTiO3 using a combination of density functional theory and interatomic potentials. A common approach used for this type of study is “mode following”: starting from the high temperature cubic phase, lower symmetry phase can be assessed by following the eigenvectors of the imaginary phonon modes (if they are away from the Brillouin zone centre, they involve a supercell expansion). The challenge for the student was that the phase changes are delicate, with meV energy changes that test the limits in the accuracy of the methods and the precision of the codes.
I have written about hybrid perovskites before. The operation of replacing an atomic A site in a perovskite by an isovalent molecule makes matters even worse for materials modelling: the space group operations of standard perovskite are lost. For CH3NH3PbI3 (MAPI), even starting from a cubic basis, the deformation of the PbI3 cages around the molecule are large*. There are also no standard symmetry constrains to stop a tetragonal phase becoming orthorhombic (most molecules break the a = b lattice vector equality for any static configuration). An additional complication is the librational-rotational disorder of the molecules at temperatures relevant to solar cells. Reassuringly, the physically correct behaviour is recovered from molecular dynamics simulations [initially reported here]:
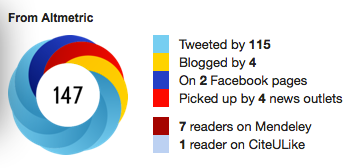 Of course this can be a useful tool for tracking engagement (or massaging your ego), but not as a serious metric for quality or impact as many are now proposing (and some institutions implementing). I was disappointed to receive an email this week from the journal
Of course this can be a useful tool for tracking engagement (or massaging your ego), but not as a serious metric for quality or impact as many are now proposing (and some institutions implementing). I was disappointed to receive an email this week from the journal